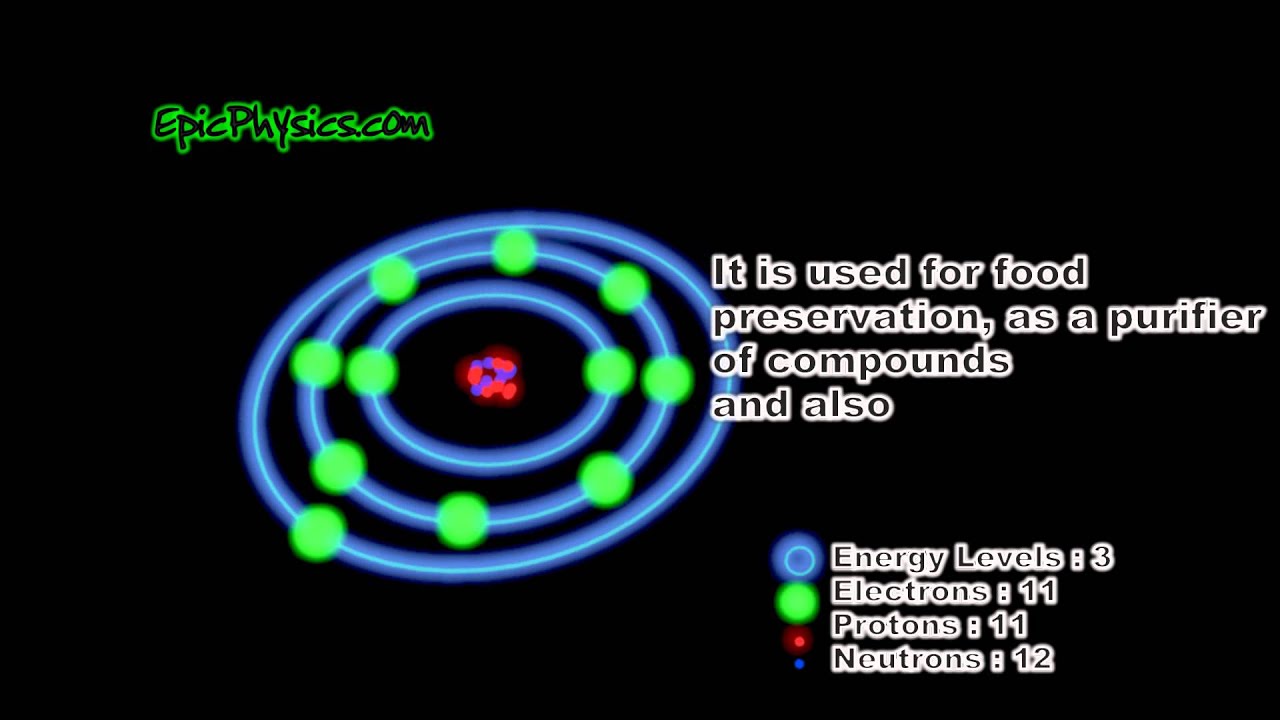
Sodium Facts & Bohr Model YouTube
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.

[DIAGRAM] Board Diagram Of Sodium
Bohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an atom, they can be useful to help with visualizing electrons orbiting a nucleus. Drawing Bohr-Rutherford diagrams is super easy using the following steps:

Bohr Diagram Of Sodium Atom model, Atom diagram, Atom model project
How to Draw the Bohr-Rutherford Diagram of Sodium chemistNATE 252K subscribers Subscribe Share 56K views 4 years ago Sodium has 2 electrons in its first shell, 8 in its second and 1 in.

Bohr Rutherford Diagram For Sodium
Bohr model of Elements. 1. Hydrogen (H) 1. 2. Helium (He) 2. 3. Lithium (Li)
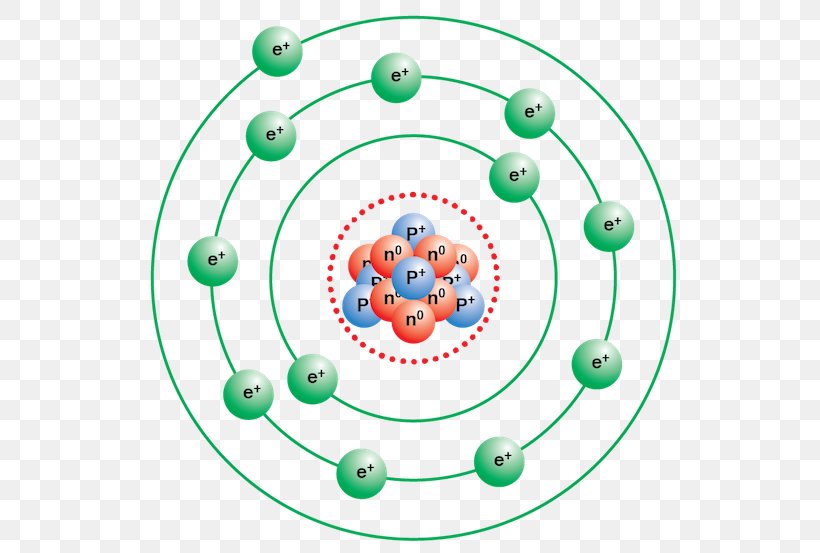
Bohr Model Sodium Atom Chemistry Rutherford Model, PNG, 550x553px, Bohr
1. Find the number of protons, electrons, and neutrons in the Sodium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom

This is the electron configuration for sodium. Like ALL of the other
Figure 1 RUTHERFORD-BOHR DIAGRAM OF AN OXYGEN ATOM . Science and technology 404 Chapter 1 ATOMS AND ELEMENTS Page 4 SAMPLE QUESTIONS: 1) Oxygen is a gas important for life and it represents about 21% of the Earth's atmosphere.. Sodium Hydrogen Fluorine Neon 6) Draw a Rutherford-Bohr atomic model for each of the following elements. Write.
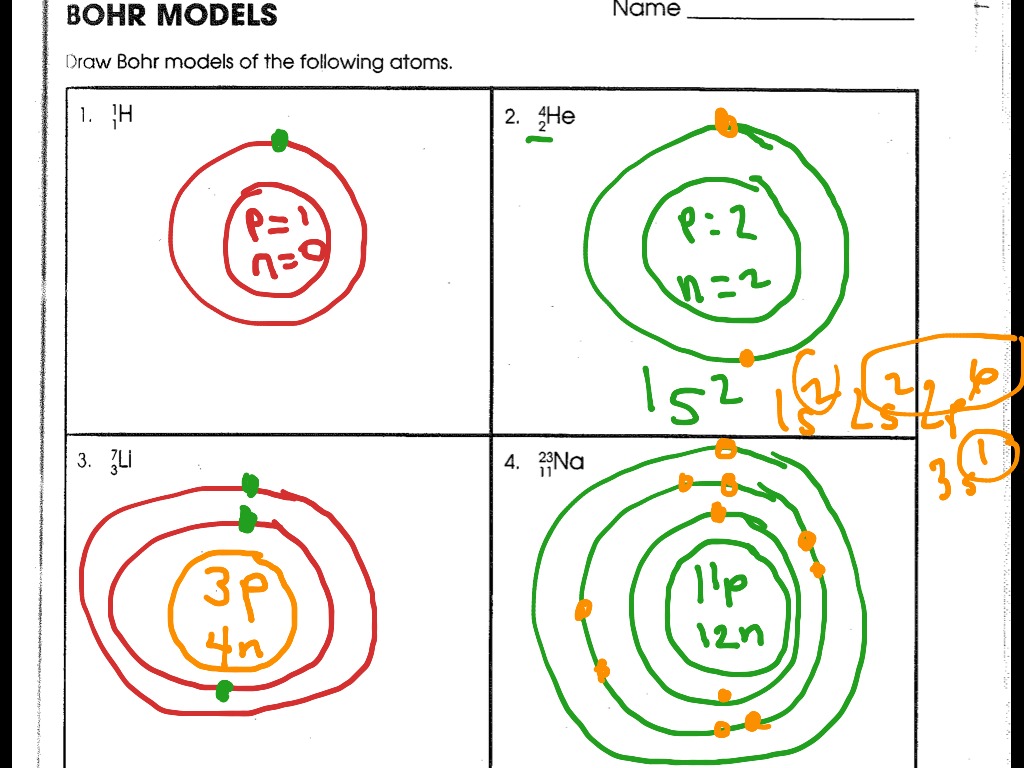
Diagram Bohr Model Periodic Table Periodic Table Timeline
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.

Sodium Bohr Diagram
Figure \(\PageIndex{1}\): A Bhor's model can be used to diagram the location of electrons in each energy shell for an atom. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. Example \(\PageIndex{2}\) Draw the Bohr's model for sodium (Na).
[Solved] Draw a Bohrrutherford model of the ionic bond between sodium
(a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.

Bohr Diagram Of Sodium
Bohr diagram or Bohr rutherford diagram describes the visual representation of orbiting electrons around the small nucleus. It used different electron shells such as K, L, M, N…so on.

Bohr Rutherford Diagram For Sodium
Contents show Bohr Model of Sodium The Bohr-Rutherford model was given in 1913 after incorporating the findings of Niel Bohr in the already given Bohr model. The Bohr model is a representation of the atomic structure along with all the atomic particles in pictorial form.
Bohr Rutherford Diagram For Sodium
In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Bohr's model required only one assumption: The electron moves around the nucleus in circular orbits that can have only certain allowed radii.

Draw a sketch of Bohr's model of a sodium atom. Brainly.in
Steps1. Use the PT to locate atomic # and mass #2. Determine the amount of p, e and n3. Draw the nucleus placing p and n4. Place the electrons5. Draw 1st ene.
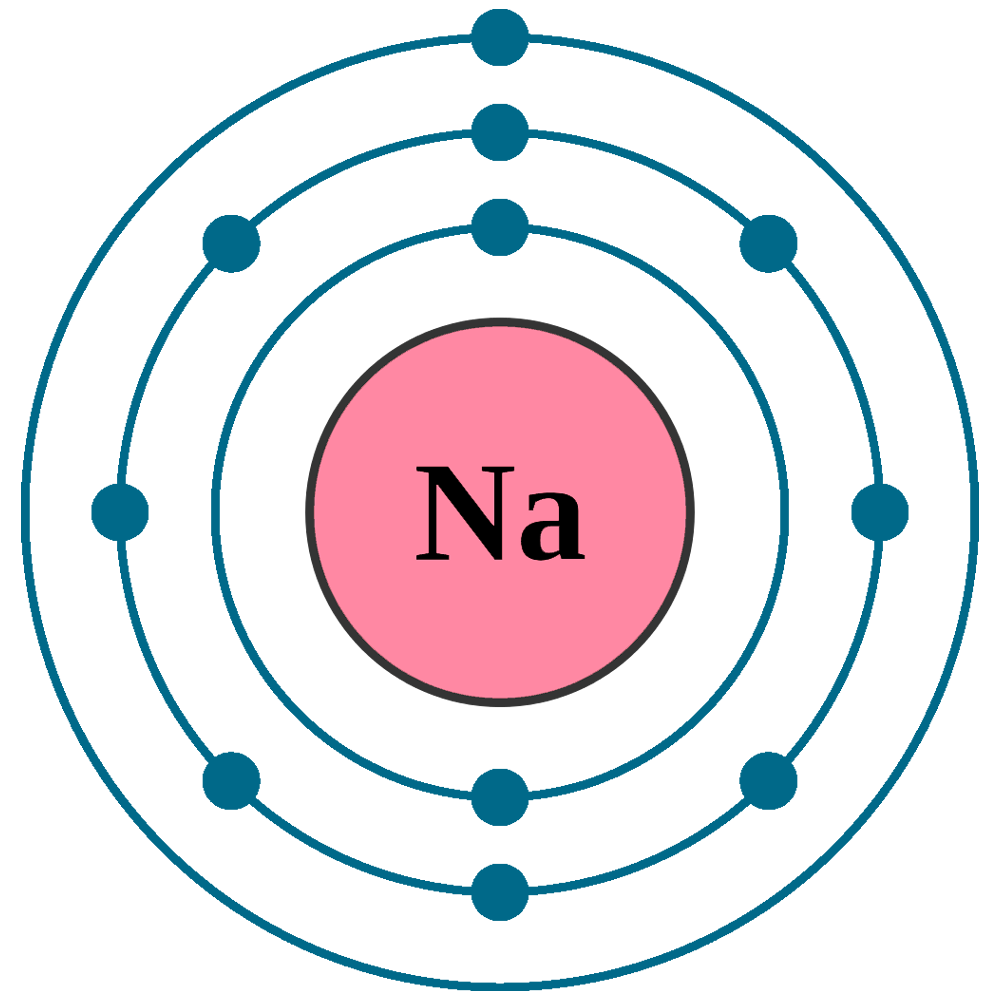
sodium electron configuration Newton Desk
The sodium Bohr Rutherford diagram is a visual representation that helps us understand the atomic structure of sodium. It is based on the Bohr model of the atom and the observations of Ernest Rutherford. This diagram shows the arrangement of electrons in the energy levels or shells around the nucleus of a sodium atom.
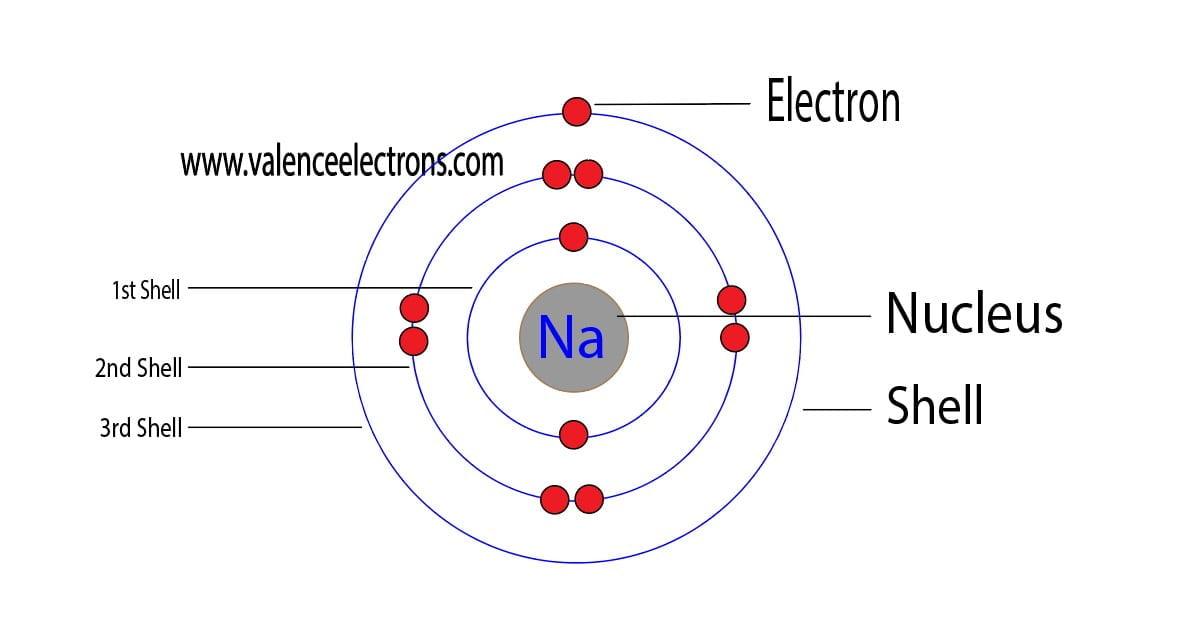
Sodium Bohr Diagram
Example 21.4.1: Emitted Photon. According to Bohr's theory two of the allowed orbits in the hydrogen atom have radii of 52.918 and 211.67 pm. Calculate the energy, the frequency, and the wavelength of the photon emitted when the electron moves from the outer to the inner of these two orbits.

Sodium Bohr Diagram
Bohr-Rutherford Diagrams. The Bohr-Rutherford Diagrams are a combinations of the atomic theories of Neils Bohr and Ernest Rutherford. They pictorially represent the atomic structure of the elements.. For sodium, it is the first element in the row, so the number of valence electrons that it has is one. If we were doing this for oxygen as a.